Purpose:
To demonstrate a chemical reaction which produces energy in the form of light without producing heat.
Materials:
-Anhydrous sodium carbonate (Na2CO3)
-Water
-Luminol (3-aminophthalhydrazide) (C8H7O2N3)
-Sodium bicarbonate (NaHCO3)
-Ammonium carbonate monohydrate ((NH4)2CO3*H2O)
-Copper (II) sulfate pentahydrate (CuSO4*5H2O)
-3% Hydrogen peroxide (H2O2)
-Flask
-Funnel
-Spiral condenser or plastic tubing
Procedure:
1)Clamp the spiral condenser to the ring stand. Attach a funnel at the top to pour the solutions into, and place a flask at the bottom.
2)Prepare solution A in a 500-mL flask:
3)Add 250-mL of water to the flask.
4)Dissolve 2.0g of anhydrous sodium carbonate.
5)Add 0.1g of luminol and dissolve. (Luminol may take a
long time to dissolve.)
6)Add 12.0g of sodium bicarbonate.
7)Add 0.25g of ammonium carbonate monohydrate.
8)Add .2g of copper (II) sulfate pentahydrate.
9)Stir until all the chemicals dissolve.
10)Add water until the total volume of the solution is 500 mL.
11)Prepare solution B in another 500-mL flask.
12)Add 25 mL of 3% hydrogen peroxide.
13)Add water until the total volume of the solution is 500 mL
14)Pour solutions A and B simultaneously into the funnel, and dim the room lights. As the solutions react, a blue glow results 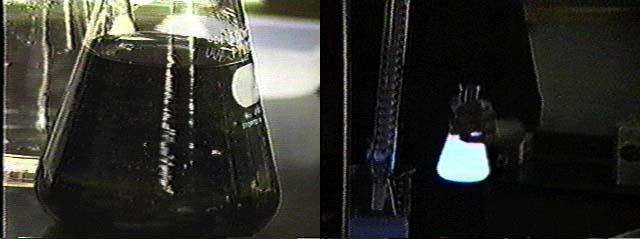
|

